Pipeline
ONX-1006
ONX-1006 Efficacy test (preliminary)
Study for Determination of ONX-1006 Concentration in AsPC-1 Pancreatic Cancer Xenograft Model
| Group | Dose(mg/kg) | PTX loading(mg/kg) | |
|---|---|---|---|
| G1 | Negative control | 0 | 0 |
| G2 | ONX-1006 (low dose) | 20 | 1.84 |
| G3 | ONX-1006 (mid dose) | 50 | 4.6 |
| G4 | ONX-1006 (high dose) | 100 | 9.2 |
| G5 | Paclitaxel group | 20 | 20 |
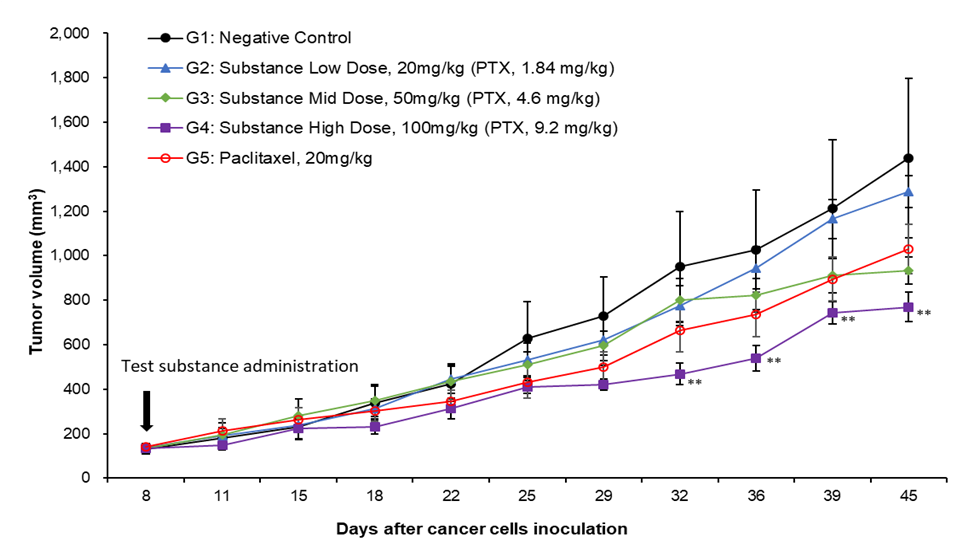
Figure 1. Tumor volume.
Test substance IV injection on Day 8, 15, 22, 29 and 36
Significant difference from the Negative control group by Dunnett's t-test: ** p < 0.01
Data presented mean ± S.E. (n=4)
Test institute : KFDA Certified Company, Dt&CRO Co., Ltd
Test Results : Breakthrough results and efficacy demonstrated in efficacy tests conducted by injections ( G3&G4 ).
→ Proven much superior efficacy even when administered at a dose of less than 1/2 to ¼ compared to the single dose of paclitaxel
During clinical observation No significant adverse symptoms, No significant adverse symptoms in clinical chemistry. No change in weight and coagulation.
ONX-1006 administered animals are normal - No harmful or toxic side effects observed.
ONX-1006 Efficacy test
ONX-1006 AsPC-1 Pancreatic Xenograft Test, 2021
| Group | Dose(mg/kg) | PTX loading(mg/kg) | |
|---|---|---|---|
| G1 | Negative control(Vehicle) | 0 | 0 |
| G2 | Test substance 1 (low dose) | 68 | 10 |
| G5 | Test substance 1 (low dose) | 68 | 10 |
| G7 | nab-paclitaxel | 20 | 20 |
| G8 | paclitaxel | 10 | 10 |
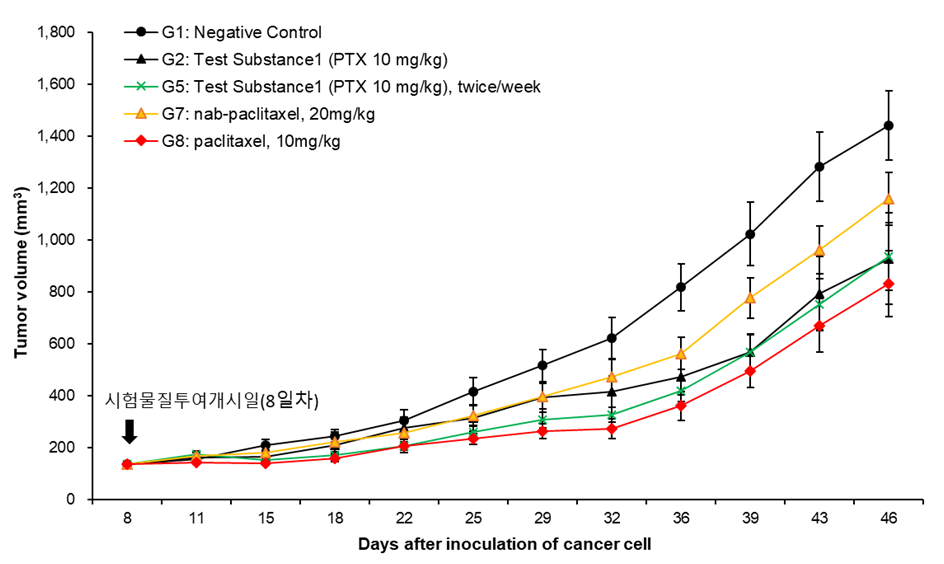
Test institute : KFDA Certified Company, Dt&CRO Co., Ltd
Test Results: Breakthrough results and efficacy demonstrated in efficacy tests conducted by injections ( G2&G5 ).
→ Proven superior anticancer effect than nab-paclitaxel, which is more advanced than existing anticancer drugs.
During clinical observation No significant adverse symptoms, No significant adverse symptoms in clinical chemistry, No change in weight and coagulation.
ONX-1006 administered animals are normal-No harmful or toxic side effects observed.
ONX-1006: PANC-1 Pancreatic cancer Xenograft Test 2020
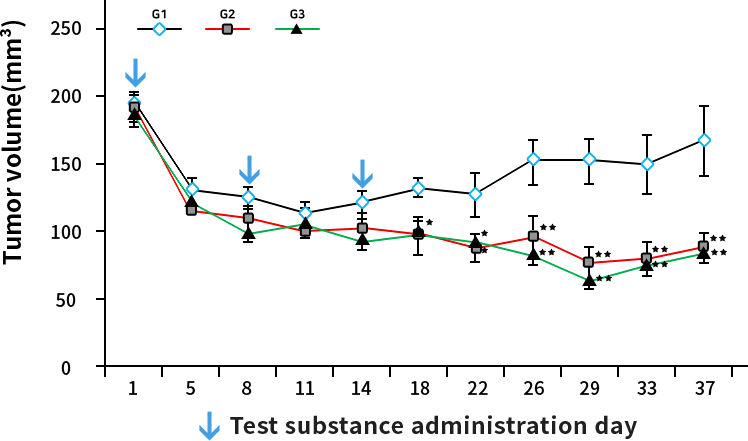
The results were statistically analyzed by ONE-WAY ANOVA * : Significantly different from G1 p<0.05 ** : Significantly different from G1 p<0.01 G1 : Vehicle control (n=9) G2 : Test article 1200 mg/kg (n=9) G3 : Test article 1900 mg/kg (n=9)
Test institute : KFDA Certified Company, ChemOn Inc.
Results: Breakthrough efficacy results
G2: Paclitaxel 42mg/kg: x4 RAT lethal dose
G3: Paclitaxel 67mg/kg: 6x RAT lethal dose
※ Paclitaxel (PTX) RAT lethal dose:10~12mg/kg
During clinical observation No significant adverse symptoms, No significant adverse symptoms in clinical chemistry. No change in weight and coagulation.
ONX-1006 administered animals are normal - No harmful or toxic side effects observed.
ONX-1006: MDA-MB-231 Breast Xenograft Efficacy test in 2021
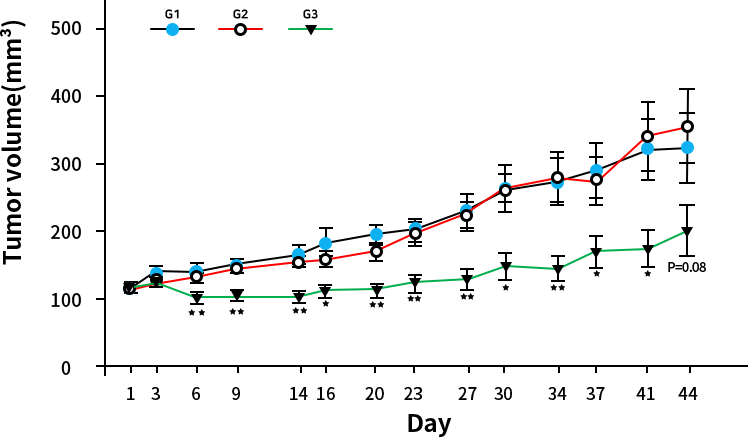
The results were statistically analyzed by Student’s t-test. * : Significantly different from G1, p<0.05 ** : Significantly different from G1, p<0.01 G1 : Vehicle control (Saline, n=10) G2 : Test article (ONX-1006, 100mg/kg, n=10) G3 : Test article (ONX-1006, 400mg/kg, n=10)
Test institute : KFDA Certified Company, ChemOn Inc.
Results: G3 Breakthrough efficacy results
During clinical observation No significant adverse symptoms, No significant adverse symptoms in clinical chemistry, No change in weight and coagulation.
ONX-1006 administered animals are normal - No harmful or toxic side effects observed.

